Which of the Following Represent the Lewis Structure for Mg
X-kr 1 Determine the electrongeometry moleculargeometryand polarity of XeF6 s A egtrigonal bipyramidal mgsee-saw polar r Begtetrahedral mgtetrahedral polar. A step-by-step explanation of how to draw the MgCl2 Lewis Dot StructureFor MgCl2 we have an ionic compound and we need to take that into account when we dra.

Is Mgcl2 Ionic Or Covalent Techiescientist
Which of the following represent the lewis structure for mg.

. The correct electron dot structure that represents the Magnesium fluoride molecule has been C. Hence the Lewis structure of Magnesium is Mg. Mg belongs to 3rd period 2nd group of periodic table has 2 electrons in the valence shell therefore lewis structure is.
I Ethyne ii Ethene iii Hexane. Which of the following represent the Lewis structure for Br. 4 valence electronsatom1 atom 4 H.
Q-Calculate the volume occupied by 88 g of CO 2 at 311C and 1 bar pressure. CLfg 1 E egtrigonal bipyramidal mgtrigonal bipyramidal nonpolar 2 Choose the ground state electron. A K and S b Ca and O c Al and N.
Lewis structures are only used to show covalent bonds and magnesium fluoride forms an ionic bond. The electron configuration of Mg is 1s22s22p63s23p64s2. It gives up two electrons.
S Mg 2. A Mg B Mg C Mg. The Lewis symbol for Mg is.
Mg can get a noble gas s2p6 configuration by losing its two 4s electrons and forming a magnesium ion Mg2. A MgN B Mg 3 N 2 C MgN 2 D Mg 2 N. Magnesium fluoride doesnt have a Lewis structure.
Part A Which of the following represent the Lewis structure for Mg. The Lewis structure of MgCl2 is therefore. O Mg 0 Mg.
Use Lewis symbols to show electron transfer between the following atoms to form cations and anions. S 4 Lewis theory predicts that the formula of a compound formed between bromine and aluminum is. Use the lewis theory to determine the chemical formula for the compound formed between Al and O.
Link shows the Lewis symbols for the elements of the third period of the periodic table. Lewis symbols illustrating the number of valence. Which of the following represents the Lewis structure for Ca2 Ca.
Complete the following four tasksquestions by labeling the diagrams above. As a general rule of thumb my chem teacher. Submit Request Answer Part A Which of the following represent the Lewis structure for Ca2.
Which of the following represents the Lewis structure for Mg. Which of the following represent the Lewis structure for Mg. Use Lewis theory to determine the chemical formula for the compound formed between Rb and O.
The correct Electron Configuration of Magnesium is 1 s2 2 s2 2 p6 3 s2. GN Lewis introduced symbols to represent the valence electrons in the atomsThey are called Lewis symbols where valence electrons are shown as dots surrounding the symbol of atom or ion. By using an expanded octet for the sulphur atom in thionyl chloride SOCl 2 you can write a Lewis structure with no formal charge.
Give the number of valence electrons for SO42-32. Option C represents the Lewis structure of Magnesium. Up to 256 cash back OneClass.
4 A Al 2 Br B AlBr 2 C AlBr 3 D AlBr. Therefore it has a total 12 electron. However considering that Lewiss condition is not as severe the doctor prescribes a daily dosage of 5 mg.
Chemistry questions and answers. How does calcium obey the octet rule when reacting to form compounds. 3 Which Lewis structure below correctly represents the compound formed between magnesium and sulfur.
S 2 D Mg. For a molecule we add the number of valence electrons on each atom in the molecule. The electron configuration of Cl is 1s22s22p63s23p5.
Hence option C is correct. How would you convert the following compounds into benzene. Which of the following represents the lewis structure for Ca 2.
Let us determine the Lewis structures of SiH 4 CHO 2 NO and OF 2 as examples in following this procedure. We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. Q-Assign oxidation number to the underlined elements in each of the following species.
A NaH 2 PO 4 b NaHSO 4 c H 4 P 2 O 7 d. Lewis is prescribed Uroxatral for the treatment of benign prostatic hyperplasia. Complete the Lewis structure for piperine and capsaicin by showing all lone pairs of electrons.
A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons. Submit Request Answer Part A Which of the following represent the Lewis structure for Br. Cl can get a noble gas s2p6 configuration by gaining an electron and forming a chloride ion Cl-.
Represent the following ionic compounds by Lewis structures. Each Uroxatral tablet contains 10 mg of alfuzosin and the common daily dosage is one tablet. Use Lewis theory to determine the chemical formula for the compound formed between Mg and N.
Atomic number of Magnesium Mg is 12. The electrons have been represented with the dot thereby the structure has been termed to be the dot structure. Up to 24 cash back The ring structures in piperine and capsaicin are shorthand notation each point where lines meet represents a carbon atom.
Lewis electron dot structure has been used for the representation of the valence electrons and the bonded electrons in the compound. So Magnesium has 2 valence electron in 3s. Determine the total number of valence outer shell electrons in the molecule or ion.
S C Mg 2. 3 A Mg. S 2 Mg B.
R 0083 bar L K 1 mol 1. Ca2 O Ca. If true enter 1 else 0.
Ca 2 Give the complete electron configuration for Ca 2 1s2 2s2 2p6 3s2 3p6.

Electron Configuration And Lewis Dot Diagrams Ppt Download
What Is The Lewis Structure For Magnesium Bromide Quora

Write Lewis Dot Symbols For Atoms Of The Following Elements Mg Na B O N And Br

How To Draw The Mgcl2 Lewis Dot Structure Youtube

5 1 Lewis Symbols And Structures General College Chemistry I
How To Draw The Lewis Dot Structure For Mgf2 Magnesium Fluoride Youtube
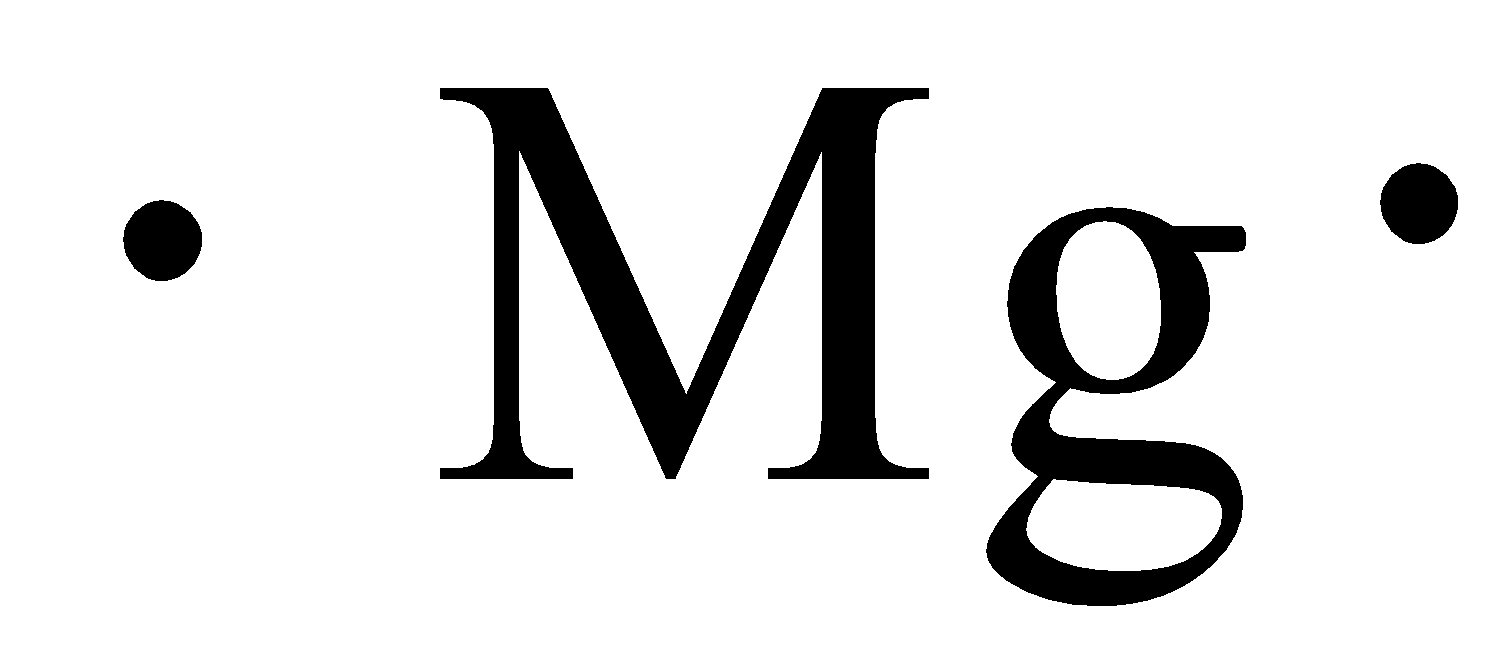
Write The Electron Dot Structure For Magnesium And Class 11 Chemistry Cbse

High School Chemistry Lewis Electron Dot Diagrams Wikibooks Open Books For An Open World

What Is The Lewis Dot Diagram For Magnesium Quora

Draw The Lewis Structure Of Mgbr2 Magnesium Bromide Youtube

File Lewis Dot Mg Svg Wikipedia

How To Draw The Mg2 Lewis Dot Structure Youtube

5 1 Lewis Symbols And Structures General College Chemistry I

Electron Diagram Of Magnesium Electron Dot Structure For Electrons Electron Configuration High School Chemistry

How To Draw The Lewis Dot Structure For Mg3n2 Magnesium Nitride Youtube
Does It Matter Where You Put The Dots On A Lewis Structure Quora

Lewis Dot Structures Dots Are Arranged Around An Element S Symbol To Indicate The Valence Electrons Reminder Valence Shell Only Has S P Orbitals Maximum Ppt Download

Which Diagram Is The Correct Electron Dot Diagram For Magnesium Brainly Com

Comments
Post a Comment